Hendrick Health consents first patient in U.S. for heart failure clinical trial
- Category: News, Heart & Vascular, Hendrick Clinic
- Posted On:

Hendrick Health has consented the first patient in the nation for the ALLAY-HF clinical study to alleviate heart failure symptoms. The patient will continue through the enrollment process, and if he/she meets the eligibility criteria, could ultimately become part of the clinical trial.
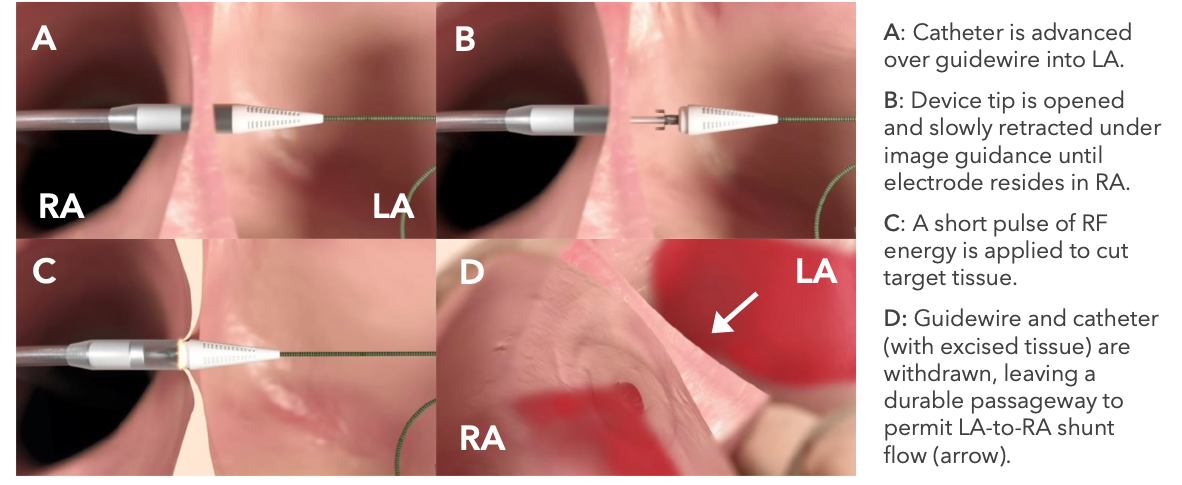
Hendrick is the first study site in the country to be initiated for the global clinical trial to evaluate safety and efficacy of Alleviant’s No-Implant Interatrial Shunt Creation for the treatment of chronic heart failure (CHF) through left atrial decompression. The study targets patients suffering from heart failure with preserved (HFpEF) or mildly reduced (HFmrEF) ejection fraction who have heart failure symptoms despite appropriate medical therapy.
“Many heart failure patients are left without options if they do not experience relief of their symptoms after significant lifestyle changes and complex medication regimens,” said Dr. Mark Lawrence, interventional cardiologist at Hendrick Health. “We are excited to explore the potential of percutaneous left atrial decompression to give these patients a chance at a better quality of life without the risk of leaving an implant behind in the heart.”
The clinical trial is double-blinded and randomized, which means neither the participants nor the researchers (physicians) know if the patients have or have not received the interventional treatment until the clinical trial ends. This makes the results less likely to be biased or be affected by factors not related to what is being tested.
The investigational medical device is delivered through a single minimally-invasive procedure without leaving a permanent implant behind. The procedure creates a connection between the left and right atrial chambers of the heart and is intended to reduce excess pressure buildup within the heart, which may reduce symptoms of heart failure such as shortness of breath, fatigue, weakness and swelling in the legs or belly.
For more information on ALLAY-HF clinical trial eligibility, visit allayhf.com.
About Chronic Heart Failure
Chronic heart failure (CHF) is a progressive disease that affects 26 million patients worldwide and is the leading cause of hospitalizations worldwide, resulting in a significant economic burden to the healthcare system. Common symptoms of CHF include shortness of breath during exertion (or at rest), inability to perform daily activities (walking, climbing stairs), fatigue and weakness, and swelling in the legs, ankles, feet or belly. Despite medications available for management of heart failure, many patients still experience debilitating symptoms, frequent hospitalization, and reduced quality of life.



